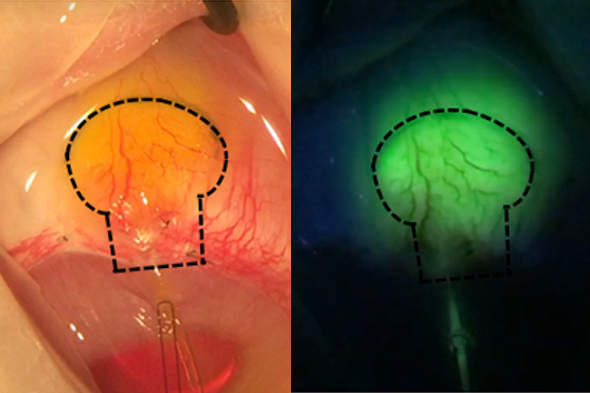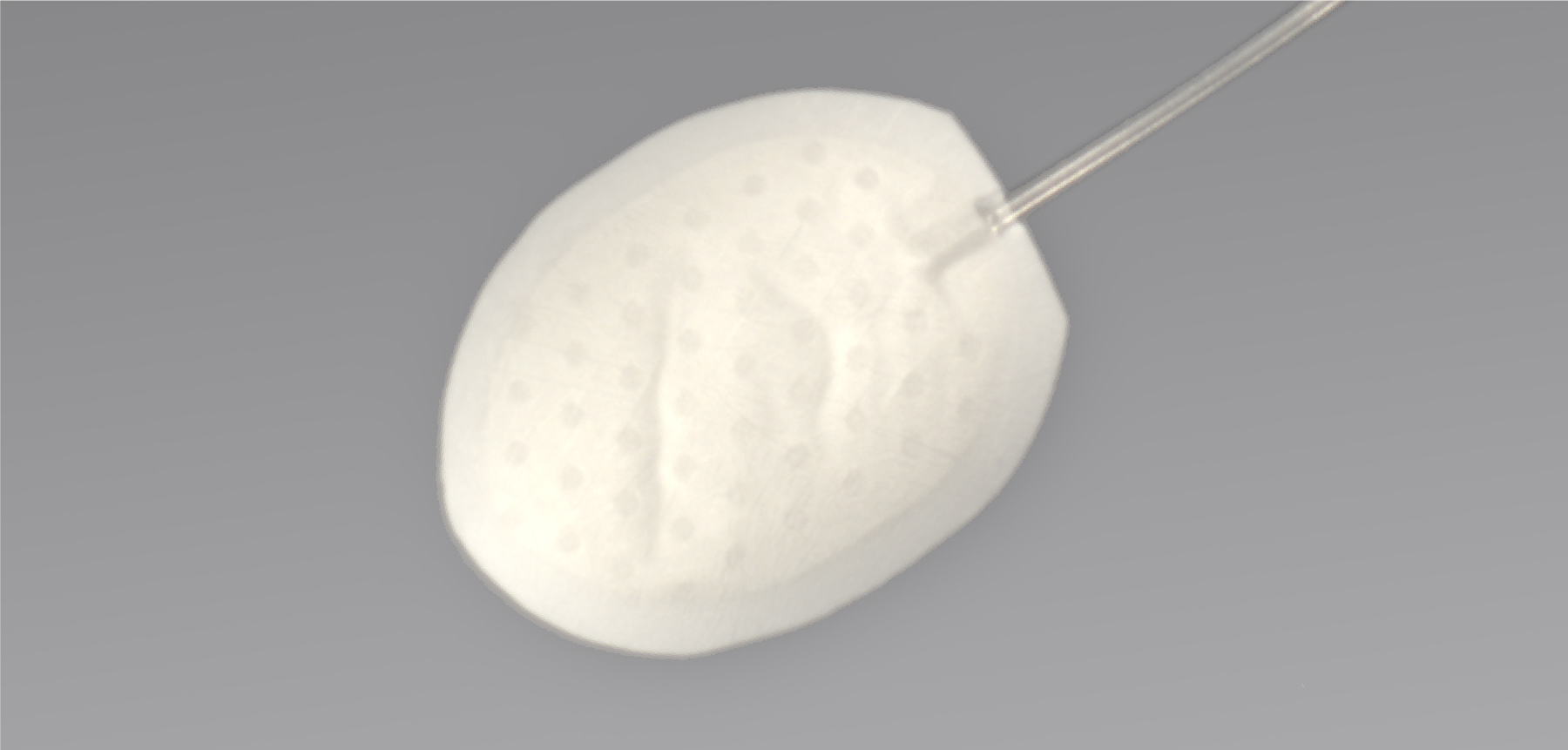
Caution: Investigational device. Not approved for use.
Investigational Glaucoma Drainage Implant
Exploring the potential of alternative surgical treatments for the leading cause of blindness worldwide.
Caution: Investigational device. Not approved for use.
Project overview
Developing an investigational glaucoma drainage device intended for use when surgical intervention is recommended.
- Will utilize a durable filtering technology with a stable biopermeable interface, intended for treating moderate-to-severe glaucoma
- Developed in collaboration with ophthalmologists and glaucoma specialists
- Feasibility studies and subject recruitment in planning stages

Tackling the leading cause of blindness
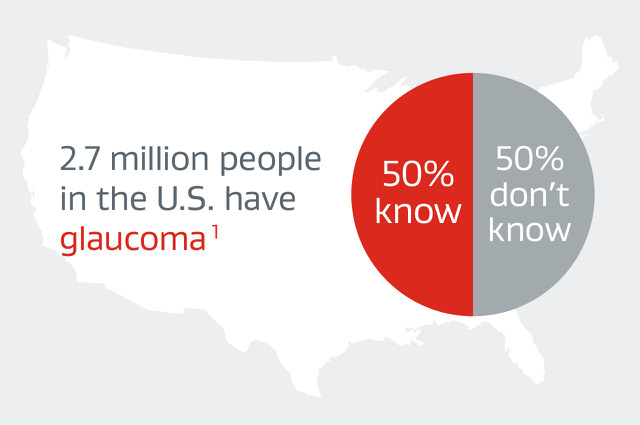
Pressure build-up in the eye from glaucoma, if left untreated, inevitably leads to optic nerve damage and vision loss.
- The most common cause of irreversible blindness, affecting 2.7 million people in the U.S. alone1
- Well managed in early stages through daily topical drug therapy but patient compliance with self-administered eye drop medication regimens is low
- Surgical intervention has high risk and failure rates (in some cases exceeding 30% after five years2), and is used only after all lower-risk, less-effective options have been exhausted
1Glaucoma [Infographic]. NIH National Eye Institute Media Library; 2013. https://medialibrary.nei.nih.gov/sites/default/files/media-images/NEI-medialibrary-2210886.jpg. Accessed October 21, 2022.
2Craven et. al. Reoperation Rates and Disease Costs for Primary Open-Angle Glaucoma Patients in the United States Treated with Incisional Glaucoma Surgery. Ophthalmology Glaucoma 2022;5(3):297-305.
Seeking a new solution for pressure relief
Investigating the possible patient benefits of using novel materials and designs may have the potential to provide an alternative to incisional procedures for moderate-to-severe glaucoma.
- The implant uses a unique fluid-permeable reservoir, providing stress relief and stable filtering
- Gore material may facilitate biointegration without use of antifibrotics or other medications indicated for reduction of tissue scarring
Additional references
- Bicket AK, Szeto J, Roeber P, et al. A novel bilayered expanded polytetrafluoroethylene glaucoma implant creates a permeable thin capsule independent of aqueous humor exposure. Bioengineering & Translational Medicine 2020;6(1):e10179.
- Bicket AK, Pitha I. Overcoming bleb encapsulation with biomaterials. Glaucoma Today 2022;2:22-25.