
Caution: Investigational device. Not approved for use.
Investigational Synthetic Cornea Device
Exploring use of established fluoropolymer materials in the design of a device intended to preserve vision when donor corneas fail.
Caution: Investigational device. Not approved for use.
Project overview
An investigational synthetic cornea device is being developed with the intent to provide alternative treatments for corneal opacity – the 4th leading cause of blindness worldwide.1
- Soft, transparent fluoropolymer optic combined with a porous, biocompatible expanded PTFE (ePTFE) skirt
- Ongoing development in collaboration with leading ophthalmic surgeons and surgical centers of excellence
- Currently undergoing preclinical feasibility testing
1Murthy_GVS, Johnson_GJ. Prevalence, incidence and distribution of visual impairment. In: The Epidemiology of Eye Disease. 3rd ed. Imperial College Press, 2012;1:3-61
The search for a viable alternative to transplantation
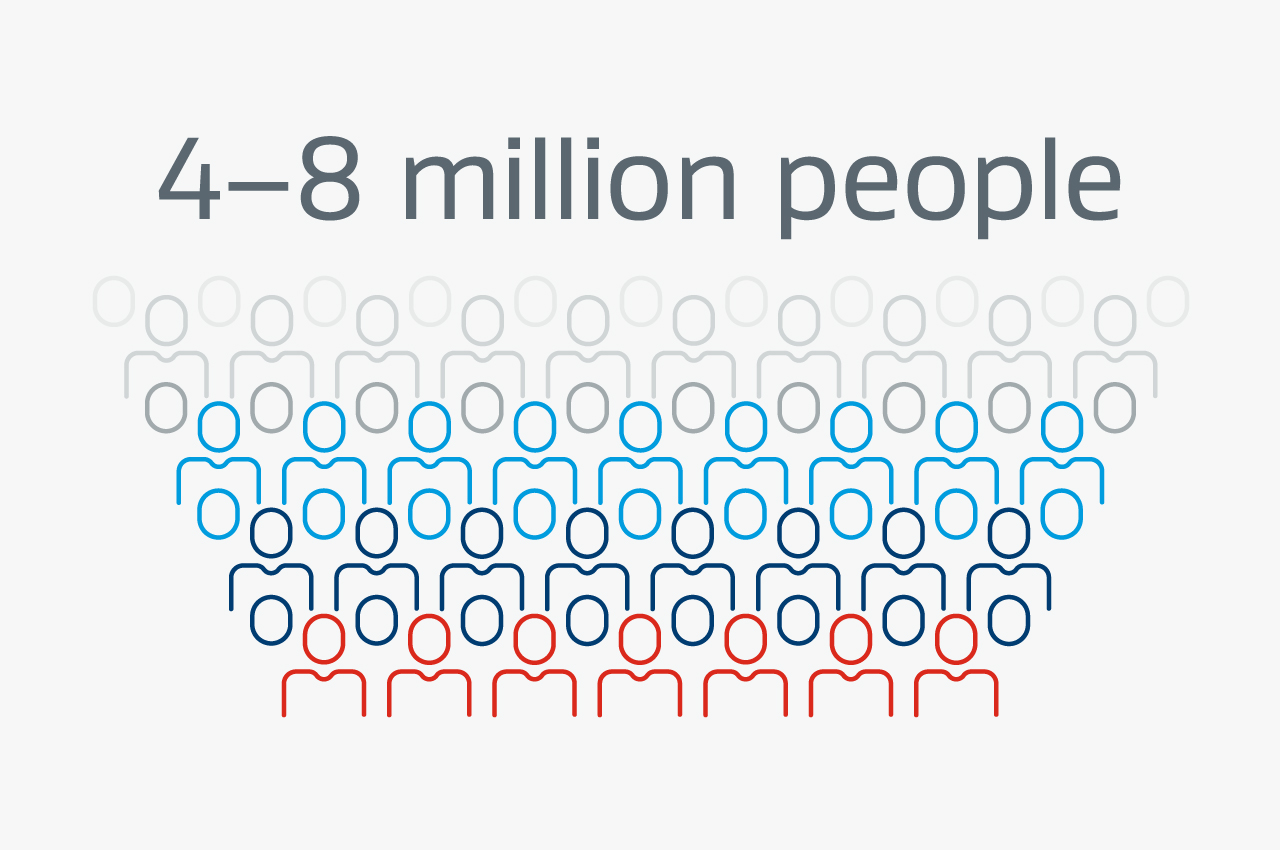
The standard treatment for corneal opacity is donor tissue transplantation, but there can be drawbacks.
- 4-8 million people worldwide suffer from blindness due to corneal opacity2
- About 10% of donor cornea transplants become opaque in a short time3
- This investigational synthetic cornea device may restore some vision where donor transplants fail
2Whitcher_JP, Srinivasan_M, Upadhyay_MP. Corneal blindness: a global perspective. Bulletin of the World Health Organization 2001;79(3):214-21.
3Ahmad S, Klawe J, Utine CA, Srikumaran D, Jimenez J, Akpek E. Survival of penetrating keratoplasty: a claims-based longitudinal analysis. Can J Ophthalmol. 2021 Feb;56(1):12-16.
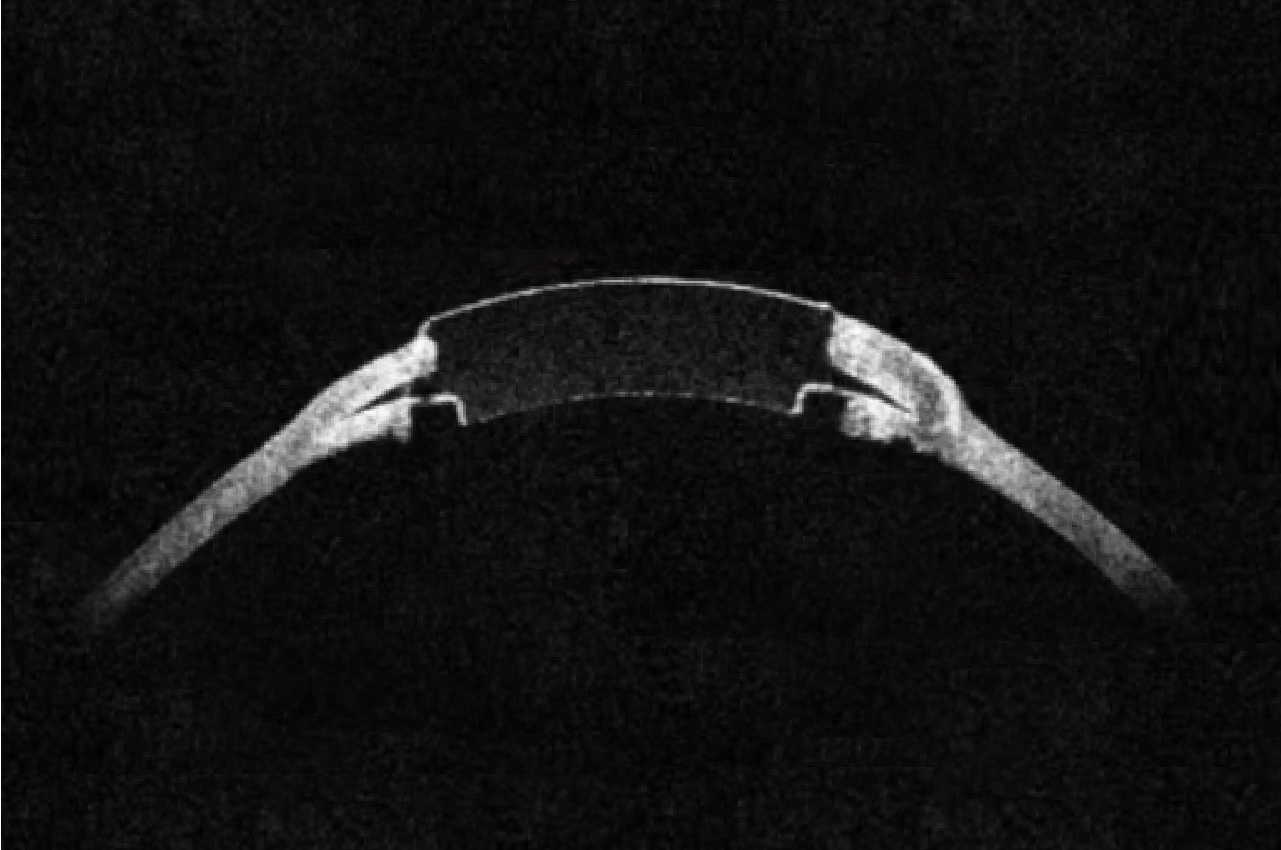
Purposefully designed with the patient and performance in mind
Gore fluoropolymer materials are designed to address the critical need for a durable cornea device
- Minimally invasive: only requires excision of a small central diseased cornea button
- Donor carrier tissue not required for device implantation
- Bioadherence of recipient corneal tissue into microporous materials observed in rabbit models
- Mimics biomechanics of natural cornea
- Low inflammation response observed in rabbit models
- Non-fouling, clear optic with built-in refractory power
- Intra-ocular pressure (IOP) measurement tested with standard clinical methods
- Designed with holes to enable flow of nutrients within the cornea
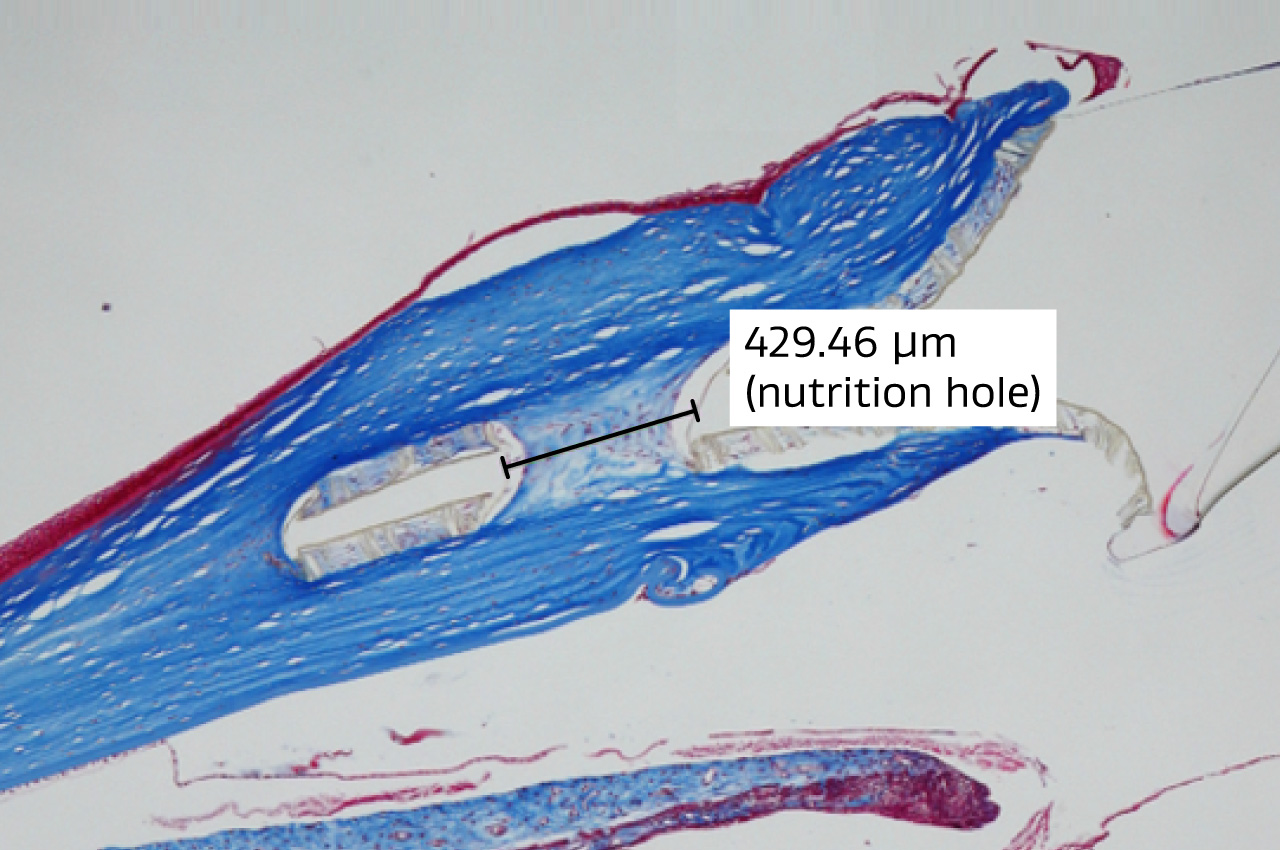
Above: Histopathology from implanted intrastromal synthetic cornea device (having nutrition hole, width measuring approximately 430um) at 3 months DPO, NZW Rabbit; Trichrome Stain